Photoredox Catalysis
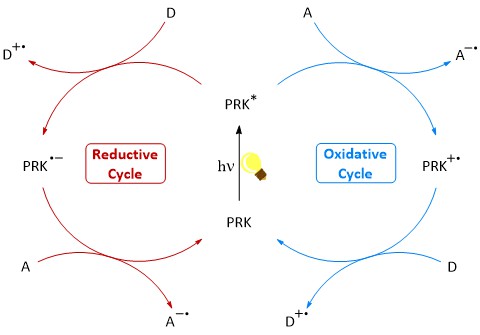
Photoredox catalysis makes it possible to activate or preserve difficult-to-access molecules under mild conditions. In this process, the so-called photoredox catalyst is excited by light, allowing an electron transfer between a substrate and itself. A second electron transfer returns the catalyst to its ground state, closing the catalytic cycle.
The most commonly used photoredox catalysts in use are based on iridium and ruthenium-polypyridyl complexes. These however, come with many of disadvantages, mainly their high cost, low sustainability and possible toxicity. In our group we focus on the development of organic chromophores as photoredox catalysts, which are inexpensive, synthetically easily accessible and less harmful to the environment. In recent years, we have worked with arylphenothiazines as highly reducing photoredox catalysts as well as naphthaline and perylene diimides as strongly oxidizing photoredox catalysts for various applications. Recently, we have also been looking into deazaflavines as potential alternatives.
We investigate our catalysts using absorption and fluorescence spectroscopy, with cyclic voltammetry and spectroelectrochemistry to evaluate their suitability as catalysts. We are currently working on photocatalytic SF6-activation, alcoxylations, C-H activation, and other projects.
For further reading, check some of our latest publications below.
N-Arylphenothiazines and N,N-Diarylphenazines as Tailored Organophotoredox Catalysts for the Reductive Activation of Alkenes
M. Giraud, M. R. Mitha, S. Klehenz, H.-A. Wagenknecht, Eur. J. Org. Chem. 2024, e202400847.
Photoredox Catalytic Access to N,O-Acetals from Enamidesby Means of Electron-Poor Perylene Bisimides
D. Steuernagel, D. Rombach, H.-A. Wagenknecht, Chem. Eur. J. 2024, 30, e202400247.
Reductive Activation of Aryl Chlorides by Tuning the Radical Cation Properties of N-Phenylphenothiazines as Organophotoredox Catalysts
F. Weick, N. Hagmeyer, M. Giraud, B. Dietzek-Ivanšić, H.-A. Wagenknecht, Chem. Eur. J. 2023, 29, e202302347.
Photochemical Activation of Sulfur Hexafluoride: A Tool for Fluorination and Pentafluorosulfanylation Reactions
D. Rombach, H.-A. Wagenknecht, Synthesis 2022, 54, 4883-4894.


.png)